-
The Future of Remote Patient Monitoring in Decentralized Clinical Trials
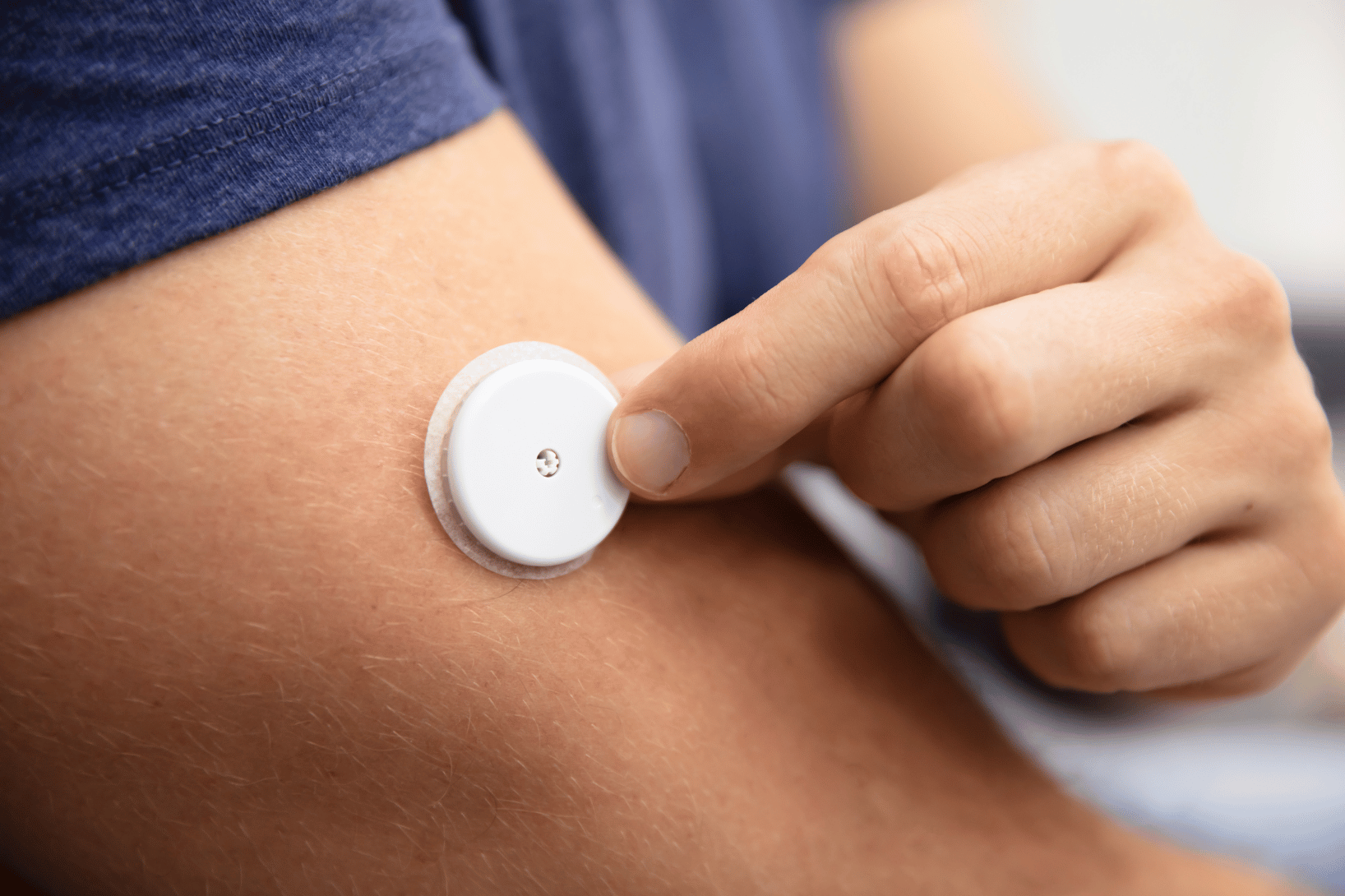
The Evolution of Remote Patient Monitoring in Clinical Trials Remote patient monitoring (RPM) has come a long way from the…
-
How Wearable Devices Improve Patient Engagement in Clinical Trials
Why Wearable Tech Is Changing Clinical Trials Wearable devices are doing more than counting steps—they’re redefining how clinical trials are…
-
Bring Your Own Device (BYOD) Clinical Trials: How They Transform Decentralized Data Capture
What Is BYOD in Clinical Trials? Bring Your Own Device (BYOD) is changing the way clinical trials collect and manage…
-
Simplifying ePRO Data Collection: The Benefits of BYOD with TrialKit
The Challenge of Device Provisioning in Clinical Trials In clinical trials, gathering timely and accurate data from patients is essential…
-
The Future is Now: Trends in Clinical Trials Brought to Reality
The trends infiltrating the clinical research industry – wearable integrations, mobile apps, machine learning, and more – aren’t just exciting concepts. They’re made possible and are now being used in studies with today’s technology.
-
Misconceptions about Bring Your Own Device (BYOD) Technology in Clinical Trials
A newer technique to the industry, bring your own device is approached with apprehension. In this post, we’ve identified four misconceptions about BYOD and provided explanations on each.