TrialKit eTMF
Manage inspection-ready clinical trial documents with confidence for faster study execution.

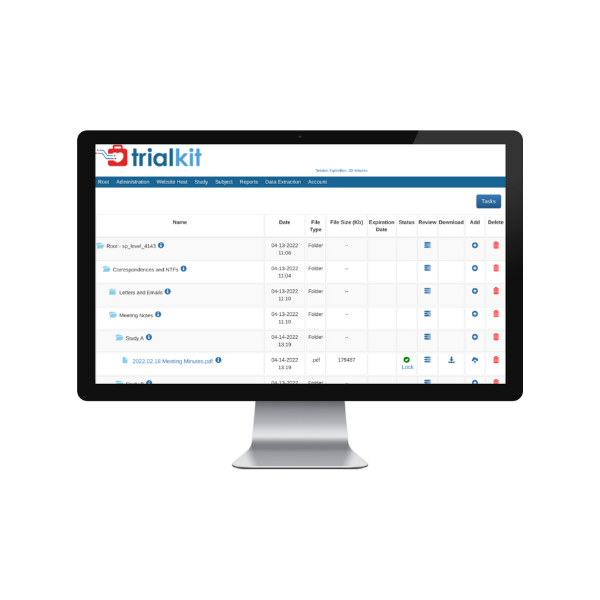

Manage your clinical trial documents in real-time for truly remote collaboration between sponsor, site, and CRO
TrialKit eTMF consolidates mission-critical documents in a central location for easy access for faster TMF delivery and study execution.
Tour TrialKit eTMF
Why TrialKit eTMF?
Move away from error-prone paper- and spreadsheet-based processes. TrialKit eTMF integrates with TrialKit EDC as well as external vendors in the cloud, ensuring your documents stay current, accurate, and inspection ready.
Stop sorting through paper and spreadsheets
TrialKit eTMF consolidates your trial documents into one central location. Your documents are easily accessible from anywhere in real-time, making remote collaboration a reality.
Get off the ground in days (not weeks)
TrialKit eTMF comes with a built-in template based on CDISC’s Trial Master File Reference Model v. 3.3.1. Combined with the drag-and-drop functionality inherent in all TrialKit products, you can implement TrialKit eTMF in days, not weeks.
Integrate with multiple vendors
TrialKit eTMF pairs nicely with the TrialKit platform. As a standalone offering with RESTful API, it plays well with other data collection and management systems and applications. Use it for multiple studies and with multiple vendors.
Designate custom workflows
Design workflows to suit your study. Create custom folder structures, change folder and file properties, and maintain regulatory data in a central location.
Secure access among sponsors and sites
Role-based permissions allow administrators to designate who can view, edit, and upload/download eTMF documents. You can also allow certain site staff and study team members to access, edit, and share essential site documents from the same repository.
Speed up trial execution
eTMF nearly eliminates risk of errors caused by misplaced or mismanaged documents. Instead, you get inspection-ready documents stored in a system that grows with your organization.
Get a complete audit trail
To ensure compliance, all transactions and changes in TrialKit eTMF are logged in a verifiable and reportable audit trail, including file versioning information.

