
Run Your Entire Study on One eClinical Platform – From Anywhere
TrialKit replaces the patchwork of systems you’ve been forced to stitch together. Clinical research software that manages your studies from startup to closeout on one platform that’s fast, affordable, and works wherever you do.



Simplify the Complex with TrialKit
TrialKit gives you everything you need to manage your studies, without the bloat. From startup to closeout, every feature is built to save time and put you in control. Access it anytime, anywhere, whether on the web, iOS or Android app, or native macOS desktop. No clunky add-ons. No surprise fees. Just a flexible, mobile-ready platform that works the way you do.
Everything You Need to Run Better Studies
TrialKit is a unified eClinical platform with all the tools you need to collect, manage, and act on your study data, whether you’re in the clinic or working remotely.
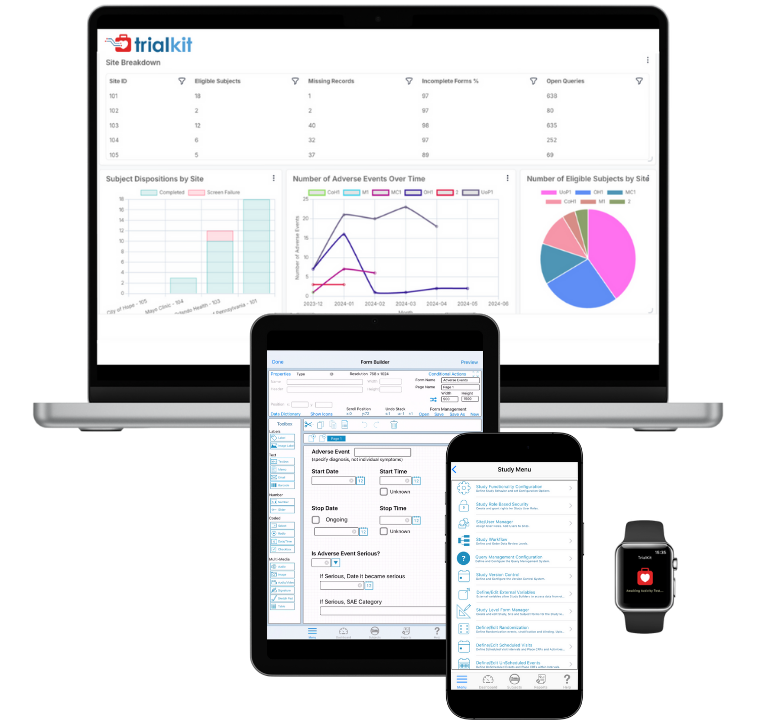
Electronic Data Capture (EDC) System for Clinical Trials | TrialKit
Build and manage studies with ease. TrialKit’s flexible, mobile-ready EDC makes it fast to set up, simple to update mid-study, and easy to maintain high data quality.
Read more →eSource & Direct Data Capture (DDC) for Clinical Trials | TrialKit
Collect source data directly at the point of care, online or offline. Eliminate duplicate entry and reduce errors with real-time, site-friendly data capture.
Read more →eCOA and ePRO Solutions for Clinical Trials | TrialKit
Let patients report outcomes from anywhere. Our mobile-friendly design ensures a smooth experience for in-clinic or remote participation across all device types.
Read more →eConsent for Clinical Trials | TrialKit
Simplify consent while staying compliant. Secure, remote-ready workflows make it easy to inform and onboard participants from any location.
Read more →eTMF for Clinical Trials | TrialKit
Organize and store essential trial documents in a secure, compliant, cloud-based system. TrialKit’s eTMF makes it easy to maintain inspection readiness with real-time access, version control, and audit trails.
Read more →Randomization & Trial Supply Management (RTSM) | TrialKit
Inventory/Trial Supply Management module for web and mobile can be configured to randomize and track subjects, drug supply, devices and inventory throughout the course of a study.
Read more →TrialKit Payment Manager
Track and manage site payments accurately and on time. Automate payment calculations, streamline approvals, and reduce administrative burden, without relying on spreadsheets or third-party tools.
Read more →Standard Reporting and Dashboards
Get instant visibility into study performance. Built-in dashboards and configurable reports make it easy to monitor enrollment, data quality, query resolution, and more. No programming required.
Read more →Clinical Adjudication Software for Clinical Trials | TrialKit
Coordinate blinded adjudication processes with ease. TrialKit’s adjudication tools support event tracking, committee workflows, and secure documentation, all in one place.
Read more →Medical Coding for Clinical Trials | TrialKit Coder
Simplify medical term standardization with integrated support for WHODrug and MedDRA coding. TrialKit enables efficient, compliant coding workflows that reduce errors and improve data consistency.
Read more →EHR to EDC Solutions for Clinical Trials | TrialKit
First-of-its-kind technology using our proprietary machine-learning API to transform unstructured EHR or other data into usable data, helping you streamline workflows and improve data accuracy at the source.
Read more →Televisits for Decentralized Clinical Trials | TrialKit Engage
Bring the visit to the patient. Built-in video conferencing allows for remote assessments, improved accessibility, and higher retention, without switching platforms.
Read more →PACS Imaging Software | TrialKit PACS
Upload, store, and view DICOM images right inside TrialKit. No separate imaging platform needed. Just secure, compliant access when and where you need it.
Read more →AI Analytics for Clinical Trials | TrialKit AI
Make smarter decisions faster. Built-in AI surfaces trends and risks in real time so your team can respond before small issues become major delays.
Read more →
Built for Researchers Who Want Control Without the Chaos
TrialKit helps sponsors and CROs of all sizes do more with less. Whether you’re running a single trial or managing a complex pipeline of global studies, you need tools that match your pace: fast, flexible, and focused on results. With TrialKit, you can:
Customize and launch studies quickly – no developers required
Capture and manage study data with precision
Enable mobile access for remote teams and participants
Monitor performance and spot issues in real time supported by AI-powered analytics
Integrate with your existing systems, not replace them
TrialKit Solves the Problems That Slow Research Down
You’ve tried platforms that overpromise and underdeliver. You’ve dealt with siloed systems, surprise fees, and support that disappears when you need it most.
Here’s what TrialKit does differently:
One Platform, Every Function
No more juggling logins or stitching systems together. From EDC and ePRO to eConsent, reporting, and patient engagement, TrialKit keeps your entire trial running smoothly in one place.
The Industry’s Only Fully Native Mobile App
Run your study from anywhere, on any device. TrialKit’s mobile-friendly design means true access and flexibility, whether you’re in the field or working from home.
Affordable Without Cutting Corners
Enterprise-level features. Transparent pricing. TrialKit gives you the power of big systems, minus the bloated costs.
Real-Time Visibility
Know everything going on with your study the moment it happens. AI-driven dashboards and reports keep your team proactive and your timelines on track.
Ready to Scale
TrialKit works for your first study, and your fiftieth. It adapts to your needs, whether you’re running a decentralized trial, a global Phase III, or anything in between.
Human Support, Always
Get answers from real experts. Fast. Our responsive team helps you solve problems, not just submit tickets.
Who Uses TrialKit?
Researchers, sponsors, and CROs who are:
– Tired of overpaying for outdated systems
– Looking to streamline operations without adding headcount
– Ready to launch and manage studies on their terms
– Focused on data quality, speed, and compliance
– Seeking a partner, not just another vendor
40,000+
Users
9,000+
Studies
7
Continents
What Makes TrialKit Different?
We don’t sell buzzwords. We build tools that work, and we price them so you can actually use them. TrialKit brings together mobile innovation, powerful functionality, and customer-first design to support the way you really work.
We love the flexibility to modify live studies, reuse CRFs (which hastens development time for each successive study) and create stacked edit checks and conditional actions. The transparent pricing structure removes the high costs associated with launching and running studies.
Clinical Data Manager
I’ve gotten the cleanest data that I’ve ever worked with [with TrialKit].
Research Coordinator
We rely on TrialKit to document the collection of dried blood samples and upload data from users’ mobile devices to secure storage in the cloud. Collaborating with the experienced TrialKit team has been a very cost-effective method to rapidly design and test a capable and polished product.
CEO
I have built databases using SAS and Oracle Clinical, and have used many other systems. TrialKit is by far my favorite. The customer support is better than anything I’ve seen, literally, they are always just a phone call away and go above and beyond to make sure their clients are happy. TrialKit is always seeking to improve their products and are always ready and willing to hear new ideas, and often incorporate them. I love this product and support staff.
Clinical Data Manager
The customer support provided by the TrialKit team was exceptional. They are responsive, knowledgeable, and always ready to assist with any questions or concerns. Knowing I have a dedicated support team backing me up was reassuring. Overall, TrialKit has simplified and automated my trial management process, saving me time and effort while delivering excellent results. It’s a flexible tool for a start-up or larger trial, and I highly recommend TrialKit to anyone looking for an EDC to run their study. Thank you, Crucial Data Solutions, for being a great partner and support.
Head of Clinical Operations
TrialKit is an affordable, flexible, easy to use, integrated system. It allows us to reduce study build times and helps us to deploy study databases in a more timely manner for our clients. The support staff is extremely responsive and knowledgeable, and they are always seeking ways to improve their products. We have a very collaborative working relationship with the team at CDS.
SVP Resourcing Operations







