-
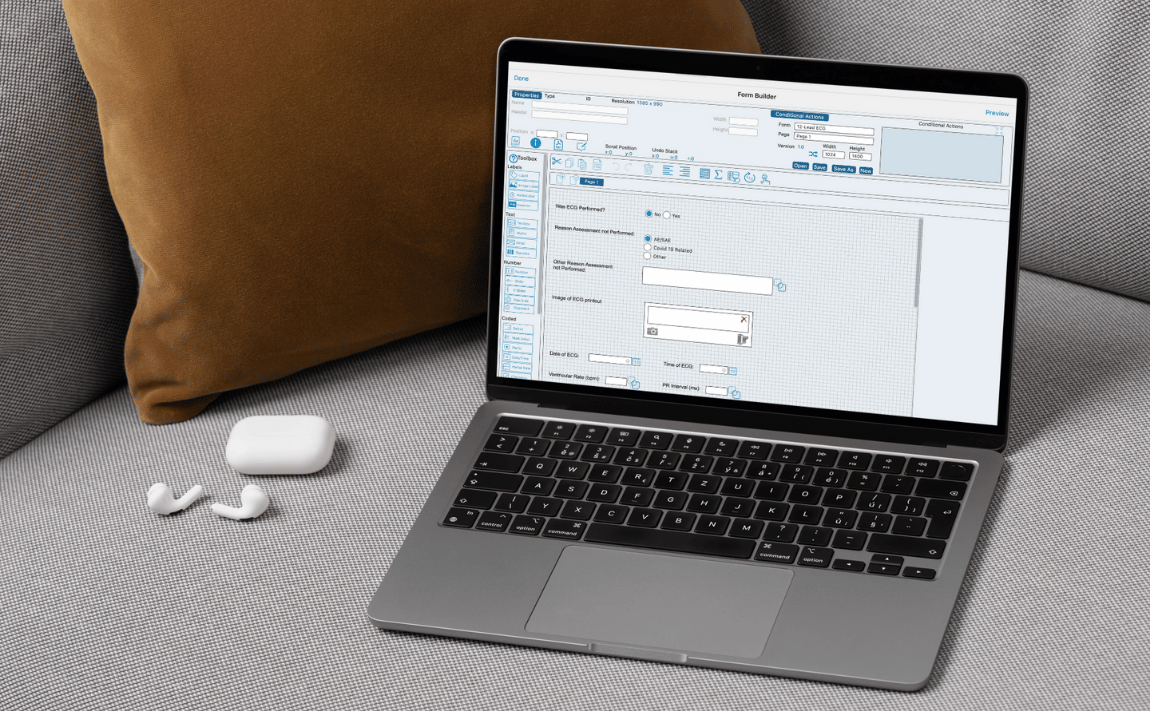
Modern Post-Market Trials Will Be Driven by RWD – Is Your EDC Ready?
The Post-Market Shift: From Sites to Data Streams Phase IV trials used to mean tracking long-term outcomes by continuing to…
-
Smarter Clinical Trials for CNS and Pain: AI Analytics and Objective Measurement Tools
Clinical trials in pain and neurological research have historically faced steep hurdles: lengthy timelines, high costs, and subjective data endpoints…
-
Ensuring Regulatory Compliance When Using Wearables in Clinical Trials
Regulatory Risks of Wearables in Clinical Trials Are Real (and Avoidable) Wearables have become indispensable in modern clinical trials, tracking…
-
Crucial Data Solutions Sets Another Industry Benchmark: TrialKit Now Available as a Native macOS App
The only eClinical platform to offer native web, mobile (iOS/Android), and Mac applications, redefining the standard for modern research software….
-
The Future of Remote Patient Monitoring in Decentralized Clinical Trials
The Evolution of Remote Patient Monitoring in Clinical Trials Remote patient monitoring (RPM) has come a long way from the…
-
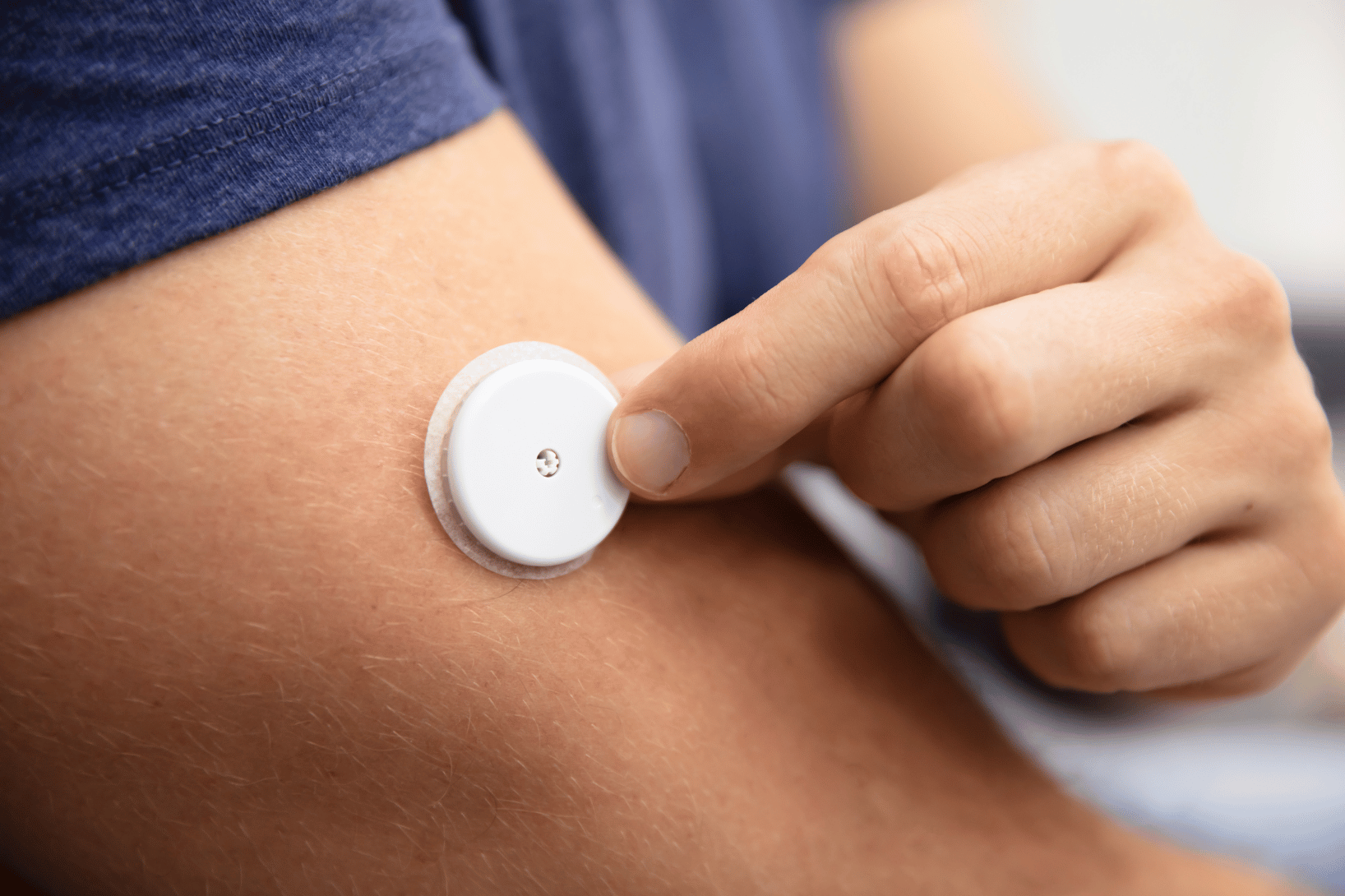
How Wearable Devices Improve Patient Engagement in Clinical Trials
Why Wearable Tech Is Changing Clinical Trials Wearable devices are doing more than counting steps—they’re redefining how clinical trials are…
-
Crucial Data Solutions Launches Industry-First Subscription Model to Modernize Clinical Trial Contracting and Pricing
New model empowers CROs to grow revenue, reduces costs for sponsors, and accelerates time to market RENO, NV, UNITED STATES,…
-
Bring Your Own Device (BYOD) Clinical Trials: How They Transform Decentralized Data Capture
What Is BYOD in Clinical Trials? Bring Your Own Device (BYOD) is changing the way clinical trials collect and manage…
-
The Future of Wearable Technology in Clinical Trials
What Are Wearables and How Are They Used in Clinical Trials? Wearable technology is transforming clinical trials by making it…
-
How EDC Software Minimizes Bias in Clinical Trials
The Impact of Bias on Clinical Trials and Patient Safety Bias, despite best efforts and best intentions, continues to impact…