-
Smarter Clinical Trials for CNS and Pain: AI Analytics and Objective Measurement Tools
Clinical trials in pain and neurological research have historically faced steep hurdles: lengthy timelines, high costs, and subjective data endpoints…
-
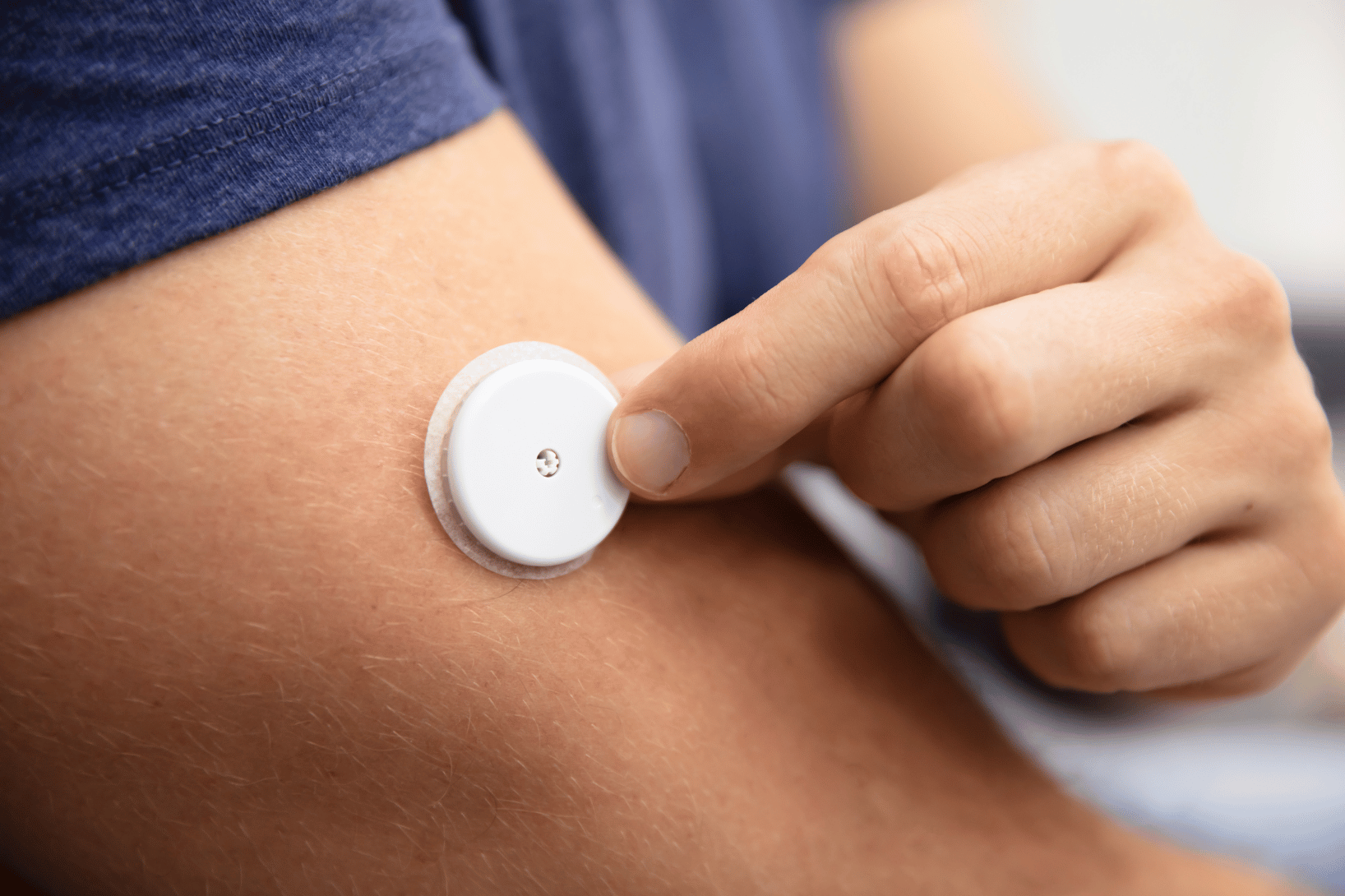
Ensuring Regulatory Compliance When Using Wearables in Clinical Trials
Regulatory Risks of Wearables in Clinical Trials Are Real (and Avoidable) Wearables have become indispensable in modern clinical trials, tracking…
-
The Future of Remote Patient Monitoring in Decentralized Clinical Trials
The Evolution of Remote Patient Monitoring in Clinical Trials Remote patient monitoring (RPM) has come a long way from the…
-
How Wearable Devices Improve Patient Engagement in Clinical Trials
Why Wearable Tech Is Changing Clinical Trials Wearable devices are doing more than counting steps—they’re redefining how clinical trials are…
-
Bring Your Own Device (BYOD) Clinical Trials: How They Transform Decentralized Data Capture
What Is BYOD in Clinical Trials? Bring Your Own Device (BYOD) is changing the way clinical trials collect and manage…
-
Patient-Centricity and Decentralized Clinical Trials
This is the first blog in a two-part series recapping our recent webinar, Ahead of the Curve: Emerging Technologies in Clinical Trials, with our partner Triall. Here, we’ll summarize our perspectives on patient centricity, decentralized trials, and shifting study participants from research subjects to informed collaborators.
-
Patient-Centered Clinical Trials: Tips for Sites
Improving the patient experience is a collective effort for all those involved in the clinical research space, but it doesn’t have to be burdensome. Here, we offer some suggestions on how to alleviate some of the challenges faced by sites – specifically surrounding eClinical technology.
-
Patient-Centered Trials are Successful Trials
At the writing of the blog post, we’re starting to witness the industry reach an inflection point when it comes to decentralized clinical trials (DCTs) and their implications for research teams. But our approach to providing eClinical technology at Crucial Data Solutions remains constant: equip researchers with the right tools so they can meet patients…