-
Accelerate Your Study Starts – How to Avoid Database Go-Live Delays with TrialKit’s EDC System
Delays in study start-up and database go-live can create significant challenges in clinical trials. First off, delays cost money – …
-
Avoiding Clinical Data Vaporware – Even More Questions to Ask eClinical Software Providers
In our last blog, we brought up the topic of vaporware in clinical research data management. Clinical trials require a…
-
Build Better Studies Faster: 3 Advantages of Mobile eClinical Platforms
The landscape of clinical research is undergoing a rapid digital transformation. Data can be collected in ways not imagined just…
-
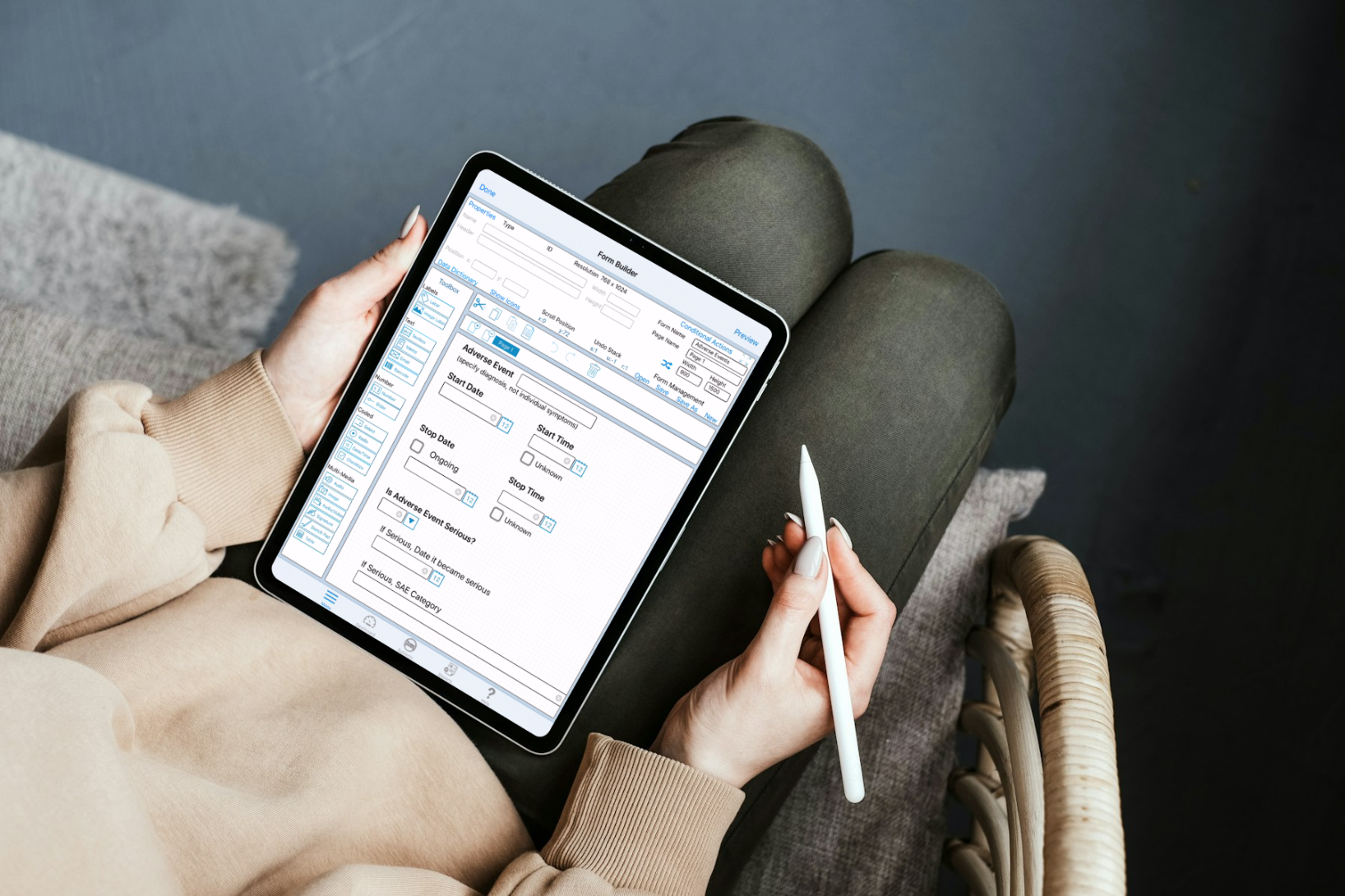
Improve Data Collection with These Tips for Electronic Case Report Form (eCRF) Design
In this post, we reveal some tips and techniques for designing powerful and purposeful CRFs that fuel accurate data collection.
-
How to Shorten the Duration of a Clinical Study Build
Shaving time off the trial design and study build process enables research teams to dedicate more resources to other important tasks. Discover some of the ways to accomplish a shorter study build in this post.
-
The Perfect Form
Crucial Data Solutions’ CEO, Paul Grady, characterizes the perfect case report form and the capabilities your software should have in order to deliver it.
-
How Responsive Forms Benefit Your Research
A highly configurable form building platform offers a number of benefits for designing case report forms. Let’s explore the ways TrialKit’s flexible form builder enhances the experience for the study designer and for the end-user.
-
Case Report Forms in Clinical Trials: Did you Ask the Right Questions?
When creating CRFs in a clinical study build, it’s important to ask the right questions, but it is equally important to ask in the right way. Let’s take a closer look at different form fields and how to leverage them to prompt more accurate responses in the data collection process.
-
5 Reasons for Sponsors to Build Their Own Study in EDC
Sponsor companies needn’t feel restricted to sourcing out their study builds to CROs. Many times, they can accomplish their own builds with the right EDC system – and save considerable resources.