-
It’s Your Data: Open API Gives You Better Access and the Freedom to Build and Manage Your Studies, Your Way
Unlike some providers in the eClinical space, at CDS we do not hold your data hostage when you conduct your studies in TrialKit. In fact, TrialKit is driven by an open API that gives users true freedom and access to their data – whenever they need it. Read on to learn how we do this…
-
Highlights of 2022 at Crucial Data Solutions
With the holidays upon us, we’ve been reflecting on our favorite takeaways from the past year – with our clients and partners, the TrialKit platform, and our team at CDS. Scroll through this post to see what topped our list of highlights from 2022.
-
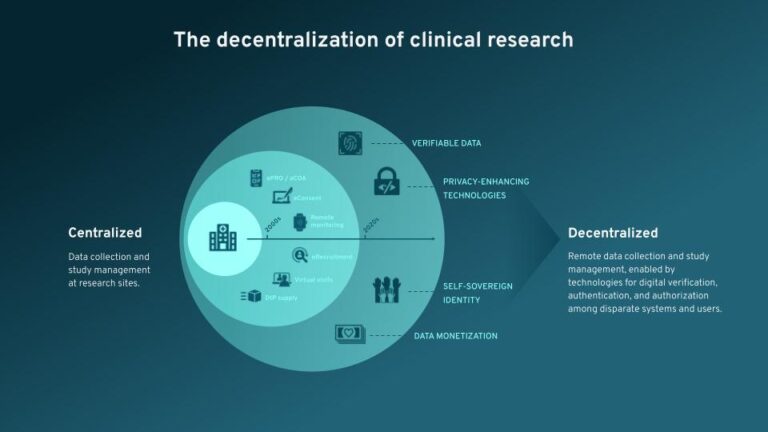
Getting Value from Emerging Technologies – Overcoming Barriers to Adoption
The second blog in this two part series recapping our recent webinar, Ahead of the Curve: Emerging Technologies in Clinical Trials, we take a look at how we can break down the barriers to adopting rising technology solutions like blockchain and SSI in clinical research.
-
Patient-Centricity and Decentralized Clinical Trials
This is the first blog in a two-part series recapping our recent webinar, Ahead of the Curve: Emerging Technologies in Clinical Trials, with our partner Triall. Here, we’ll summarize our perspectives on patient centricity, decentralized trials, and shifting study participants from research subjects to informed collaborators.
-
Leveraging Empowered Patients to Build Better Clinical Registries
It’s time to find a better way to run clinical registries that works for today’s population – without exorbitant costs and bottlenecks. In this post, we offer our own suggestions on accomplishing this and explain how it can be done right now.
-
Patient-Centered Clinical Trials: Tips for Sites
Improving the patient experience is a collective effort for all those involved in the clinical research space, but it doesn’t have to be burdensome. Here, we offer some suggestions on how to alleviate some of the challenges faced by sites – specifically surrounding eClinical technology.
-
Patient-Centered Trials are Successful Trials
At the writing of the blog post, we’re starting to witness the industry reach an inflection point when it comes to decentralized clinical trials (DCTs) and their implications for research teams. But our approach to providing eClinical technology at Crucial Data Solutions remains constant: equip researchers with the right tools so they can meet patients…
-
The Win-Win with Virtual Visits – Better Experiences, Better Data
The rising number of decentralized trials and hybrid trials over the past few years has necessitated the use of televisit solutions for remote study conduct. But there are a number of benefits to virtual visits for both research professionals and study participants, which we begin to explore in this blog post.
-
Exploring the Benefits of Low-Code/No-Code Study Design
On the fence about adopting a low-code/no-code solution for your studies? Here, we take you through three real-world experiences our users have had with TrialKit as their data collection and study management platform.
-
Getting Started with Low-Code/No-Code Clinical Trial Solutions
Have you determined that a low-code or no-code eClinical platform would be a good fit for your studies but have questions about getting started? This blog post offers some tips and considerations when searching for a solution.