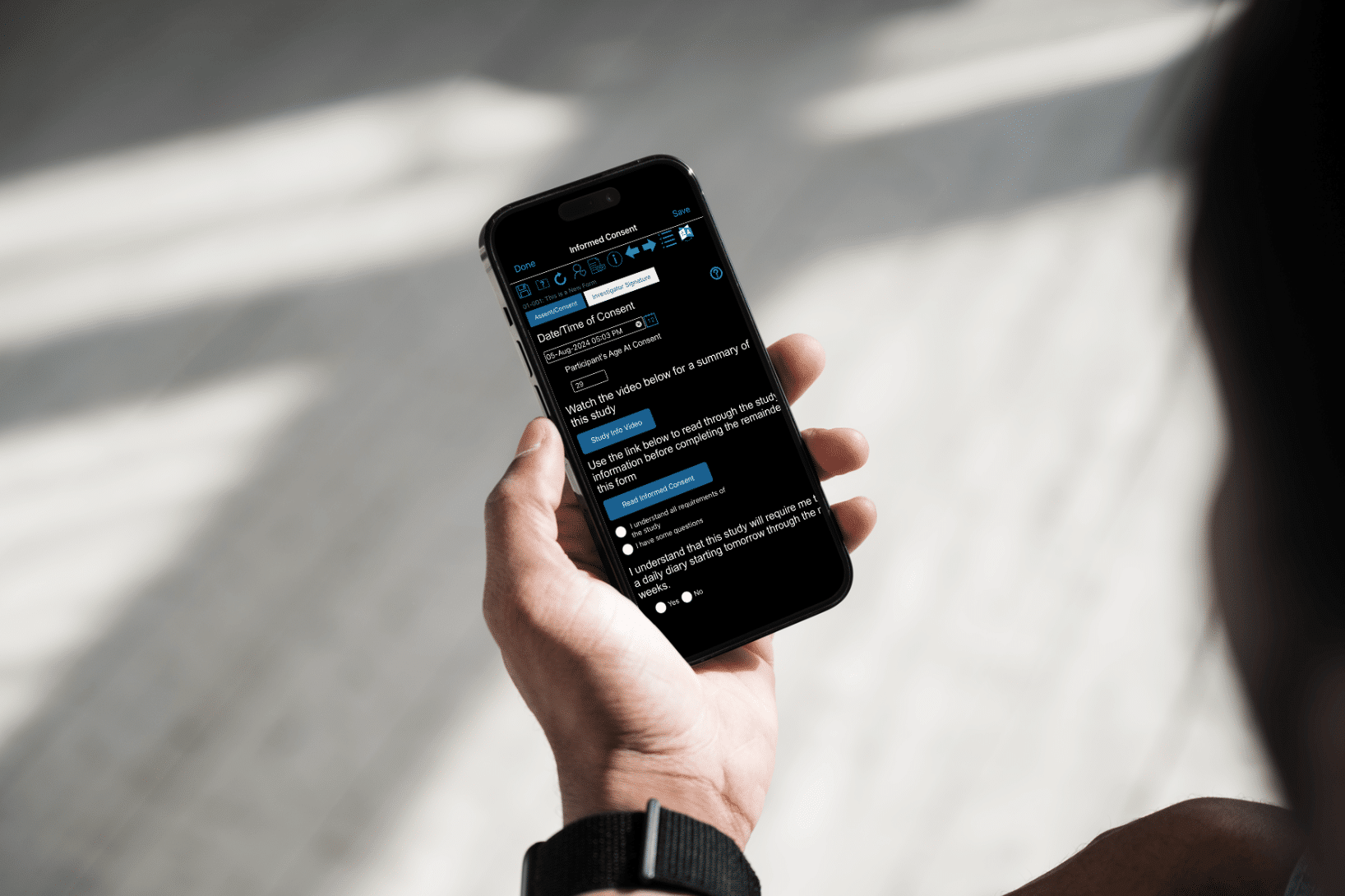
-
How to Make eConsent Accessible Across Diverse Populations
Designing Accessible eConsent for Inclusive Clinical Trials Making eConsent accessible across diverse clinical trial populations means designing consent flows that…
-
Integrating eConsent with EDC: Streamlined Workflows for Seamless Trials
In clinical research, managing multiple digital systems can create unnecessary friction. When electronic informed consent (eConsent) and electronic data capture…
-
Virtual eConsent in Decentralized Clinical Trials: Best Practices and Innovations
Clinical research is increasingly shifting from traditional, site-based studies to decentralized and hybrid models that meet participants where they are….
-
Maximizing Site Performance: The Hidden Benefits of Unified eConsent and EDC
Let’s be real: managing clinical trials is tough enough without juggling multiple disconnected systems. Yet for many research teams, that’s…
-
From Paper to Digital: How eConsent Simplifies Clinical Trials
Unsure of how eConsent should tie into your eClinical platform? This post explains just why electronic informed consent is an important component of your technology toolkit.
-
Mobile Devices and Wearables: The New Source Document
In this post, CMO Dr. Leigh Mack explains how wearable devices and smartphones will play a major role in the consent process and in patient reported outcomes as clinical trials evolve.