Electronic Data Capture (EDC) System
Modern clinical trials require modern technology. Leverage the flexibility and speed of the cloud with TrialKit.



Neither traditional nor hybrid clinical trials can operate on rigid EDC systems.
They require flexible data collection systems that support protocol amendments without downtime and accept mobile data without hassle. As a cloud-native solution, TrialKit bends to accommodate mid-study changes. And as the first full-featured EDC platform available as a native mobile app, TrialKit flexes to meet the demands of traditional, hybrid/decentralized, and completely remote clinical trials.
Give your team the freedom to build, deploy, and manage their studies, their way—weeks faster than with rigid EDCs and for a fraction of the cost of the major players.
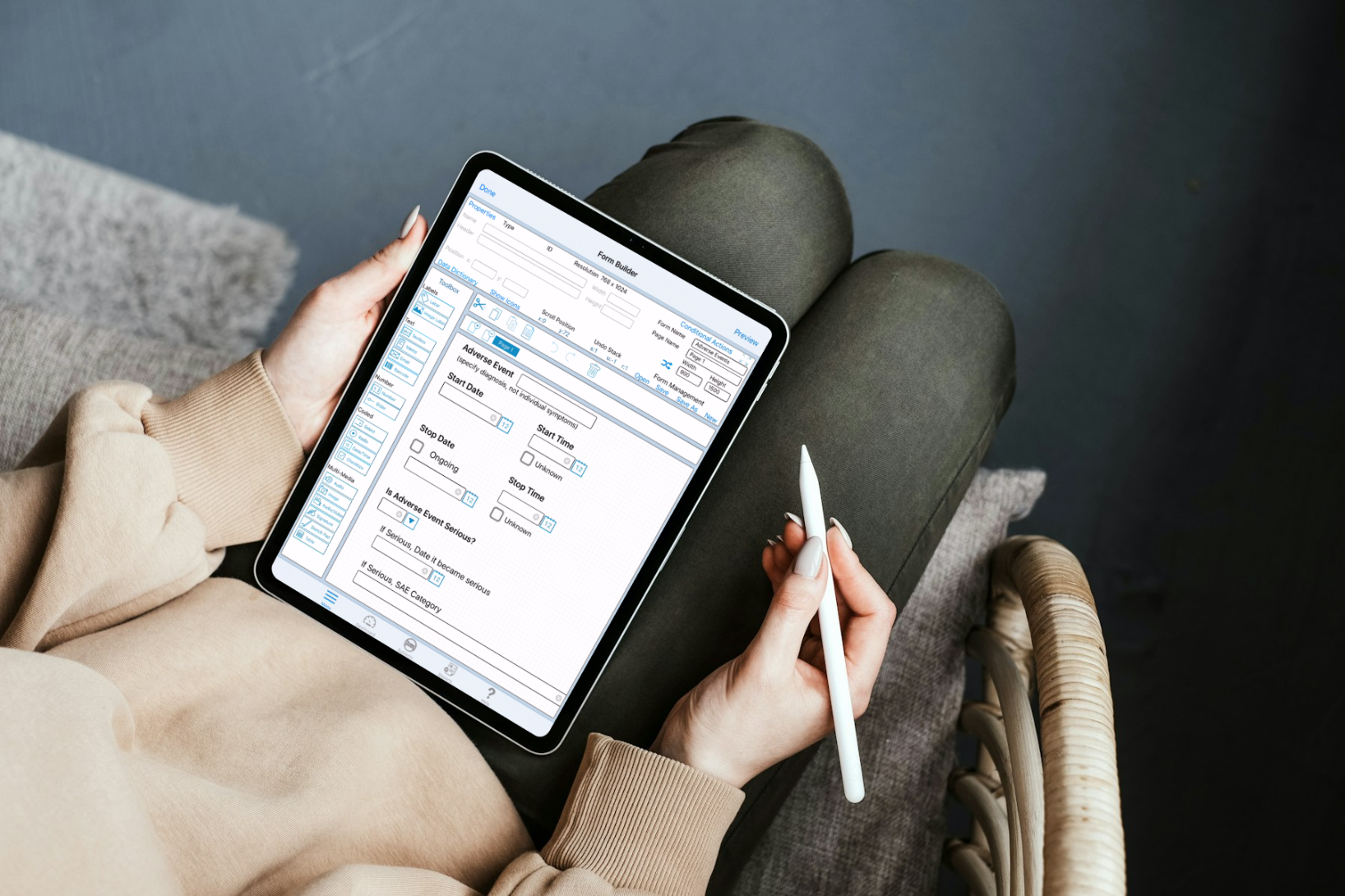
What is an EDC System?
An electronic data capture (EDC) system is a digital platform used in clinical trials to collect, manage, and store clinical data. Unlike traditional paper-based methods, which are prone to delays, transcription errors, and logistical hurdles, EDC systems enable real-time, secure data entry and monitoring from any location.
TrialKit goes beyond basic data capture. It’s a fully mobile, cloud-based system that empowers researchers to collect data directly from sites, patients, or wearable devices—anytime, anywhere. With native mobile apps for iOS, Android and Mac, TrialKit supports decentralized, hybrid, and site-based studies, setting it apart from traditional web-only EDC platforms.

Who Benefits From EDC?
Electronic Data Capture systems streamline data collection and management across a wide range of clinical research stakeholders. Whether you’re running a small pilot study or a large multinational trial, EDC systems like TrialKit can scale to fit your needs. Those who benefit include:
- Clinical Research Organizations (CROs): Simplify study builds, manage multiple trials, and ensure high data quality across sponsors.
- Pharmaceutical and Biotech Companies: Gain faster insights, improve compliance, and accelerate time to market.
- Investigators and Academic Researchers: Access an affordable, self-configurable system with powerful tools, perfect for limited budgets.
- Sponsors and Study Coordinators: Monitor progress in real time, reduce site burden, and drive operational efficiencies across global trials.
TrialKit’s flexible licensing, scalability, and end-to-end capabilities make it an ideal fit for organizations of all sizes.
Why Choose TrialKit for Your EDC Needs?
TrialKit EDC’s flexible, configurable architecture adjusts with your protocol amendments, making it easy to add cohorts and sites, modify dosing regimens, and more. Rigid systems don’t have this functionality.
Make mid-study changes with zero downtime
No more hair-puller days spent fighting your EDC. No more time-consuming migrations. Make study modifications with the click of a button.
Multiple studies, single sign-on
Sponsors and sites have controlled access to all studies—via web or mobile app—with one sign-on.
Easy eCRFs
Move field variables anywhere on the form using drag-and-drop functionality in TrialKit’s universal design tool. Create edit checks and annotated PDFs, export form data dictionaries, and more.
Reuse and repurpose forms and study builds
Save weeks off study builds by repurposing forms, variables, and conditional actions. Replicate and repurpose any study across multiple accounts from anywhere.
Secure site management
With control of 250 permissions and form access rights throughout the system, you can give sites and users only as much access as they need.
Integrated query management
Perform queries via the web or mobile app or use system-generated queries from data updated in real time. View complete audit trails to monitor study risk.
Seamless Integration with Other TrialKit Products
eSource & Direct Data Capture (DDC) for Clinical Trials | TrialKit
Collect source data directly at the point of care, online or offline. Eliminate duplicate entry and reduce errors with real-time, site-friendly data capture.
Read more →eCOA and ePRO Solutions for Clinical Trials | TrialKit
Let patients report outcomes from anywhere. Our mobile-friendly design ensures a smooth experience for in-clinic or remote participation across all device types.
Read more →eConsent for Clinical Trials | TrialKit
Simplify consent while staying compliant. Secure, remote-ready workflows make it easy to inform and onboard participants from any location.
Read more →eTMF for Clinical Trials | TrialKit
Organize and store essential trial documents in a secure, compliant, cloud-based system. TrialKit’s eTMF makes it easy to maintain inspection readiness with real-time access, version control, and audit trails.
Read more →Randomization & Trial Supply Management (RTSM) | TrialKit
Inventory/Trial Supply Management module for web and mobile can be configured to randomize and track subjects, drug supply, devices and inventory throughout the course of a study.
Read more →TrialKit Payment Manager
Track and manage site payments accurately and on time. Automate payment calculations, streamline approvals, and reduce administrative burden, without relying on spreadsheets or third-party tools.
Read more →Clinical Adjudication Software for Clinical Trials | TrialKit
Coordinate blinded adjudication processes with ease. TrialKit’s adjudication tools support event tracking, committee workflows, and secure documentation, all in one place.
Read more →Medical Coding for Clinical Trials | TrialKit Coder
Simplify medical term standardization with integrated support for WHODrug and MedDRA coding. TrialKit enables efficient, compliant coding workflows that reduce errors and improve data consistency.
Read more →EHR to EDC Solutions for Clinical Trials | TrialKit
First-of-its-kind technology using our proprietary machine-learning API to transform unstructured EHR or other data into usable data, helping you streamline workflows and improve data accuracy at the source.
Read more →Televisits for Decentralized Clinical Trials | TrialKit Engage
Bring the visit to the patient. Built-in video conferencing allows for remote assessments, improved accessibility, and higher retention, without switching platforms.
Read more →PACS Imaging Software | TrialKit PACS
Upload, store, and view DICOM images right inside TrialKit. No separate imaging platform needed. Just secure, compliant access when and where you need it.
Read more →AI Analytics for Clinical Trials | TrialKit AI
Make smarter decisions faster. Built-in AI surfaces trends and risks in real time so your team can respond before small issues become major delays.
Read more →
How an EDC System Improves Clinical Trials
An EDC system brings efficiency, accuracy, and compliance to clinical trials, transforming the way data is collected and managed. TrialKit enhances these benefits with mobile-friendly technology, real-time analytics, and unified integration with other critical study tools.
Increases Trial Efficiency
By enabling real-time, remote data entry and monitoring via mobile devices, TrialKit accelerates data collection and reduces site visits, streamlining trial workflows. Learn more.
Reduces Errors and Improves Data Accuracy
Built-in data validation checks flag discrepancies at the point of entry, ensuring high-quality data throughout the study lifecycle. TrialKit also has direct data capture (DDC) tools to minimize transcription errors. Read about integrating DDC with EDC.
Enhances Compliance
With features like audit trails, role-based access, and compliance with FDA 21 CFR Part 11, HIPAA, and GDPR, TrialKit helps ensure your trial meets regulatory requirements. Explore the basics of EDC compliance.



