TrialKit Clinical Adjudication
Consistent, unbiased, and efficient endpoint adjudication for every clinical trial

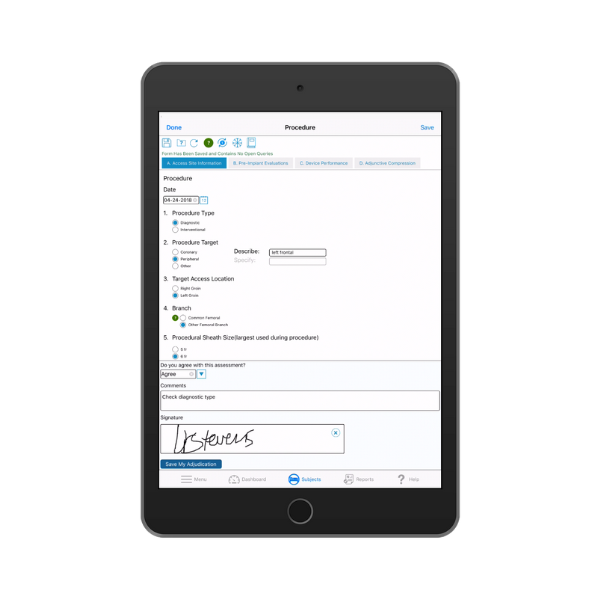

Adjudicating clinical events and endpoints is critical to trial integrity.
Bring clarity and confidence to your clinical trial outcomes with a purpose built adjudication module that supports standardized event review, reduces variability, and improves the reliability of your study results. TrialKit Clinical Adjudication centralizes committee workflows, documentation, and decisions inside a single unified platform.

What Is Clinical Adjudication and Why It Matters
Clinical adjudication provides an objective method for evaluating clinical events and endpoints by using an independent committee to apply pre defined criteria. This ensures consistency across sites, reduces investigator bias, and strengthens the accuracy of safety and efficacy assessments.
Many trials rely on adjudication because endpoints can be subjective, complex, or influenced by differences in investigator interpretation. As studies become more global and diverse, adjudication offers a reliable way to ensure uniform review criteria across all sites and patient populations.
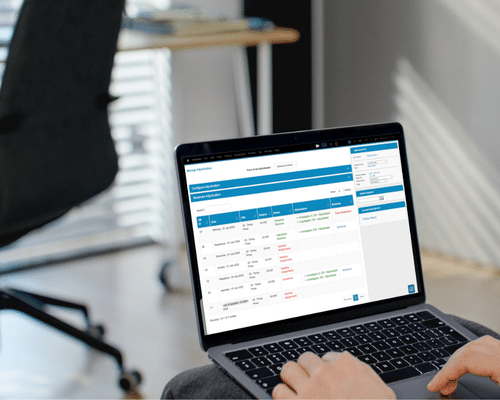
A Practical and Scalable Approach to Adjudication
Traditional adjudication processes often involve disconnected systems, manual emails, or spreadsheets that complicate committee coordination. TrialKit removes these operational barriers by providing a centralized adjudication environment that integrates directly with your study data and workflows.
With TrialKit, adjudicators, moderators, and study teams can review, classify, and track cases in a secure, organized, and fully compliant system.
Why Choose TrialKit for Clinical Adjudication
One platform for everything
Adjudication lives inside the larger TrialKit ecosystem, which includes EDC, imaging, ePRO, eConsent, and reporting. No external tools are needed for managing committee workflows or reviewing supporting documents.
Consistency and confidence in endpoint decisions
By standardizing review criteria and centralizing committee activity, TrialKit helps sponsors and CROs produce cleaner, more reliable endpoint data.
Reduced operational burden
Automated workflows, notifications, and role based permissions reduce administrative overhead and streamline complex committee coordination.
Supports both simple and complex protocols
Whether your study requires a straightforward yes or no determination or a multi step classification process, TrialKit adapts to your adjudication structure.

See TrialKit in Action
View this on-demand tutorial to learn more about TrialKit’s endpoint adjudication capabilities.
Ready to See More on Clinical Adjudication?
Request a demo to see how TrialKit Clinical Adjudication can enhance your study’s efficiency and accuracy.
