-
3 Reasons Why Business Model Matters When Selecting an eClinical Technology Partner
The news surrounding recent mass layoffs has been hard to miss and may even have you taking a closer look at the companies you choose to do business with. When it comes to evaluating vendors for your eClinical solutions, here are a few positive attributes to look for that serve as a recipe for longevity…
-
Making Data Exchange Feel Easy – Simplifying the Data Journey from EHR to EDC
Demystifying and providing a solution for EHR to EDC interoperability has been a mission for CDS for the past four years. In this blog post, we break down various interoperability challenges and how the architecture of your eClinical software can be a game-changer.
-
It’s Your Data: Open API Gives You Better Access and the Freedom to Build and Manage Your Studies, Your Way
Unlike some providers in the eClinical space, at CDS we do not hold your data hostage when you conduct your studies in TrialKit. In fact, TrialKit is driven by an open API that gives users true freedom and access to their data – whenever they need it. Read on to learn how we do this…
-
Crucial Data Solutions Sees Significant Growth Resulting from Rising Demand for Flexible eClinical Technology to Power Patient-centered Clinical Research
RENO, Nev.–(BUSINESS WIRE)– Crucial Data Solutions, Inc. (CDS), a provider of innovative low-code/no-code software to advance clinical research, saw significant…
-
Highlights of 2022 at Crucial Data Solutions
With the holidays upon us, we’ve been reflecting on our favorite takeaways from the past year – with our clients and partners, the TrialKit platform, and our team at CDS. Scroll through this post to see what topped our list of highlights from 2022.
-
Getting Value from Emerging Technologies – Overcoming Barriers to Adoption
The second blog in this two part series recapping our recent webinar, Ahead of the Curve: Emerging Technologies in Clinical Trials, we take a look at how we can break down the barriers to adopting rising technology solutions like blockchain and SSI in clinical research.
-
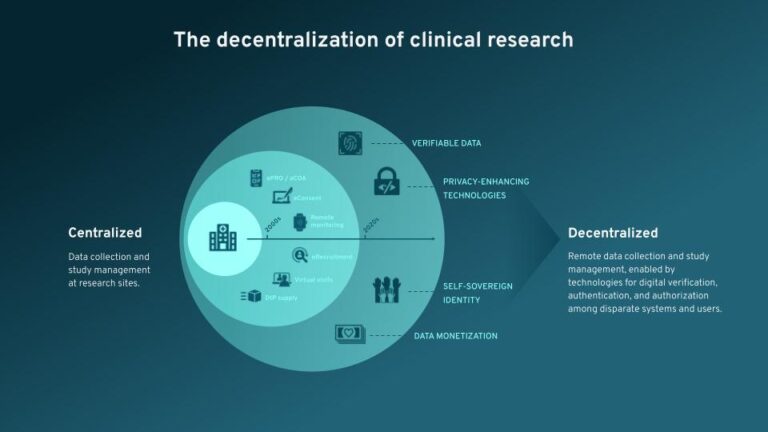
Patient-Centricity and Decentralized Clinical Trials
This is the first blog in a two-part series recapping our recent webinar, Ahead of the Curve: Emerging Technologies in Clinical Trials, with our partner Triall. Here, we’ll summarize our perspectives on patient centricity, decentralized trials, and shifting study participants from research subjects to informed collaborators.
-
Everest Group Recognizes Crucial Data Solutions as a Major Contender in Decentralized Clinical Trial Platforms PEAK Matrix® Assessment
CDS ranked as a Major Contender in the 2023 PEAK Matrix® Assessment
-
Leveraging Empowered Patients to Build Better Clinical Registries
It’s time to find a better way to run clinical registries that works for today’s population – without exorbitant costs and bottlenecks. In this post, we offer our own suggestions on accomplishing this and explain how it can be done right now.
-
Patient-Centered Clinical Trials: Tips for Sites
Improving the patient experience is a collective effort for all those involved in the clinical research space, but it doesn’t have to be burdensome. Here, we offer some suggestions on how to alleviate some of the challenges faced by sites – specifically surrounding eClinical technology.