eConsent for Clinical Trials
Electronic informed consent for patient-centric decentralized clinical trials.


Help your patients make informed decisions without burying them in paper
With electronic informed consent (eConsent) software, your patients can access consent forms using any device they choose. And because it’s part of TrialKit’s unified platform, both your patients and your study team get a seamless experience.
What is eConsent?
eConsent, short for electronic informed consent, is a digital method for delivering, reviewing, and signing clinical trial consent forms. It replaces traditional paper-based consent with a streamlined, mobile-friendly experience that improves participant comprehension and speeds up study enrollment.
Unlike static paper forms, eConsent in clinical trials enables participants to access consent materials remotely, engage with multimedia content for better understanding, and sign electronically from any device. This modern approach not only supports decentralized and hybrid trial models but also strengthens regulatory compliance through built-in audit trails and version control.
eConsent is fully integrated within the TrialKit platform, offering a seamless solution for sponsors and CROs looking to enhance participant engagement while maintaining 21 CFR Part 11, HIPAA, and GDPR compliance. Whether your trial is conducted at a site or entirely remote, TrialKit adapts to your workflow and participant needs.
TrialKit eConsent Features
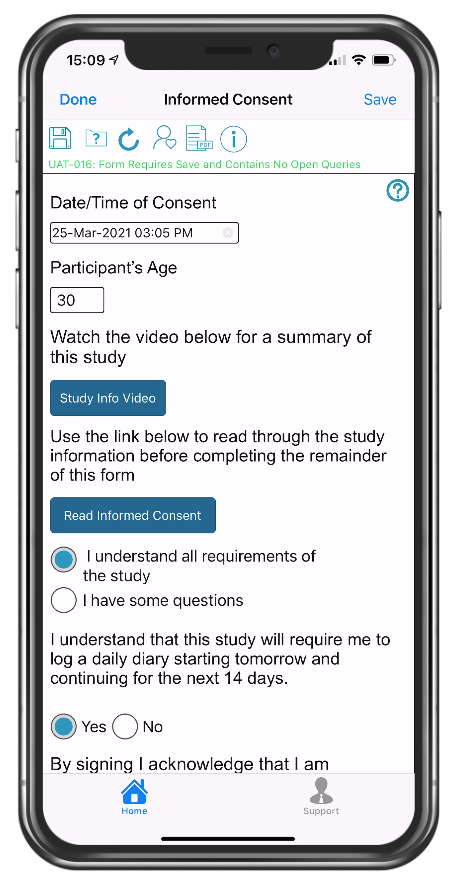
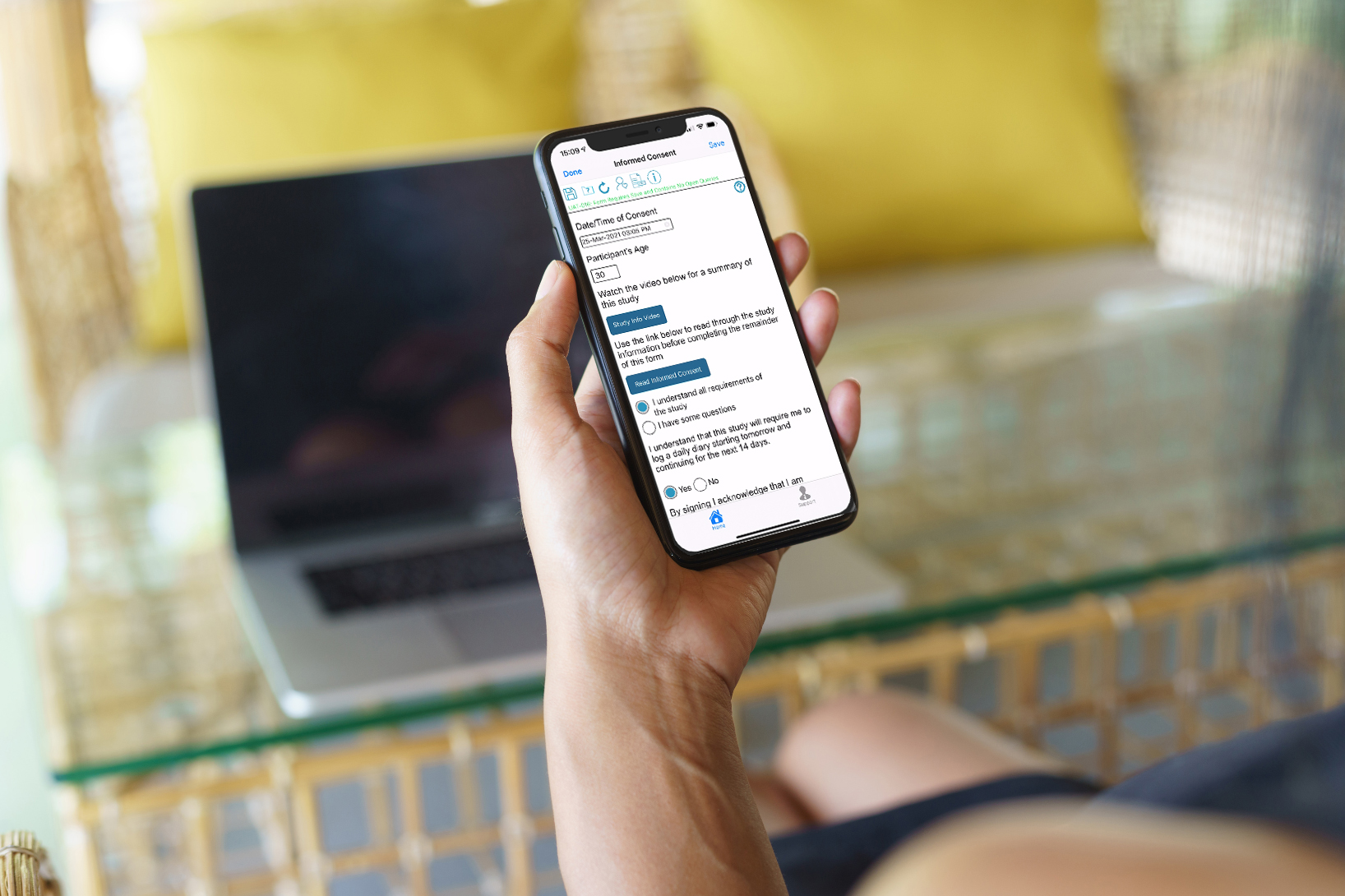
eConsent form with links to video and PDF
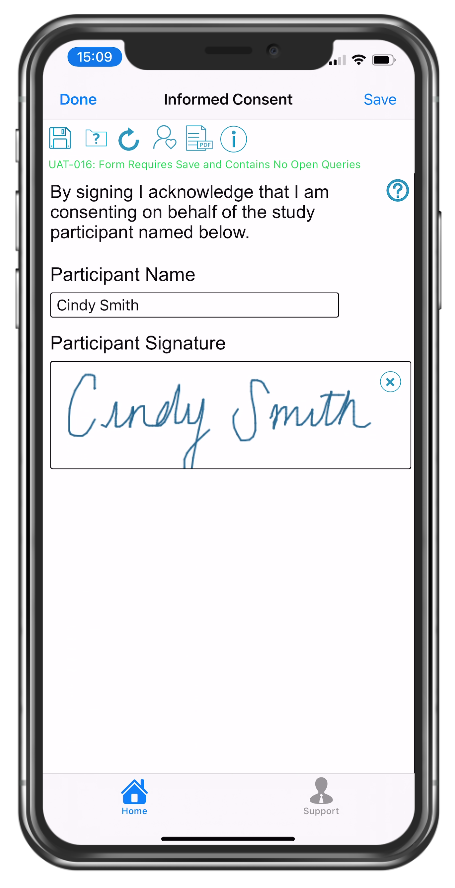
Study participant eSignature
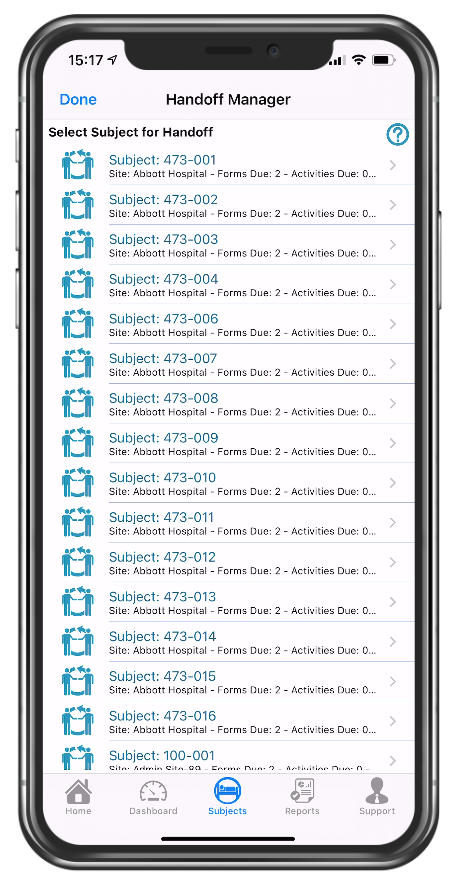
Handoff manager
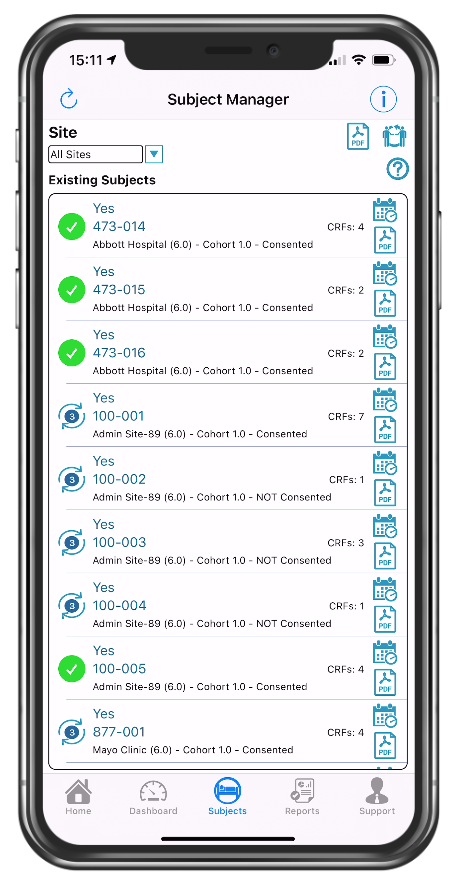
Quick view of consented participants
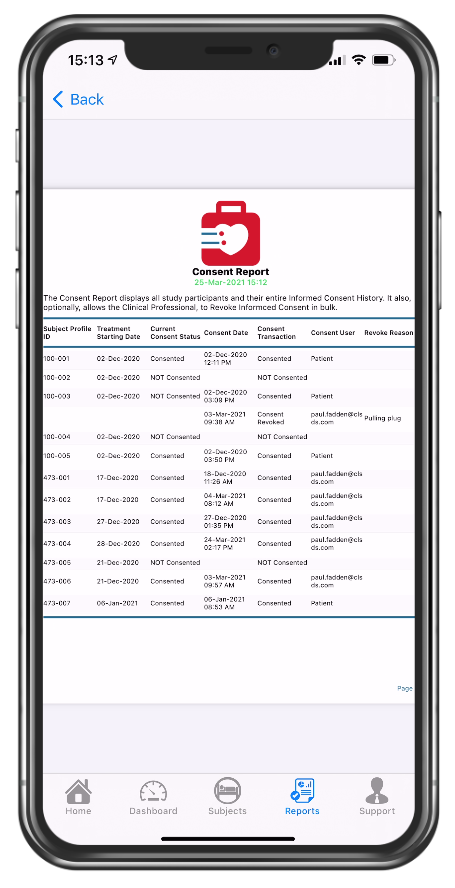
PDF of consent report
Why Choose TrialKit for eConsent?
Today’s clinical trials put patients’ needs first. TrialKit’s eConsent software makes complicated informed consent documents easier for patients to understand. And when they know what they’re signing up for, they’re more likely to enroll and stay enrolled. Fewer dropouts helps keep your study on track so you can get to market faster.
Improve patient retention
Patients receive clear explanations with educational hyperlinks, pop-up windows, photos, and videos. After consenting or reconsenting, patients automatically receive a copy of the consent forms. Improved communication and transparency leads to more committed, engaged patients.
Provide a better patient experience
eConsent allows patients to review documents at a place and time that’s most convenient – on their computer or on their mobile device. Using handoff mode, they can also complete informed consent on site with a clinician.
Run smoother global studies
Patients no longer have to visit a research site to sign a bunch of forms. They can consent from “virtually” anywhere: from home, from the office, or from a clinic. Translations in 16 languages makes your trial more accessible across ethnic groups.
Consent forms your way
Customize forms to suit patients’ needs using TrialKit’s drag-and-drop form builder. Create custom forms for each site, for pediatric and adult patients, and more. Automated email notifications keep everyone informed every step of the way.
Real-time access
Study teams can access consent data from anywhere using TrialKit’s web or mobile app. Role-based permissions keep data secure.
Improve study efficiency
TrialKit eConsent helps ensure only consented or patients move forward in your study. Automated workflows and reporting help you deploy reconsent and track which patients have provided consent. That way, you only collect data on patients who have fully executed consent.
How the eConsent Process Works
The TrialKit eConsent workflow is designed to make the consent process intuitive, flexible, and compliant—both for participants and research teams. Each step is fully digital and optimized for mobile use, allowing participants to give informed consent from virtually anywhere.
Step 1 – Participant Invitation
Participants receive a secure link via email or through the TrialKit mobile app, which allows them to begin the consent process at their convenience. Optional pre-screening steps can be included to ensure eligibility before progressing.
Step 2 – Reviewing the Consent Form
Participants are guided through an interactive consent experience that can include videos, glossary terms, and embedded Q&A sections. Built-in comprehension checks ensure participants fully understand the study requirements before moving forward.
Step 3 – Digital Signature & Documentation
Once the participant is ready, they can sign the consent form using secure eSignature authentication methods. The system automatically generates an audit-ready digital record, capturing time-stamped activity and version history for compliance and oversight.
Every step of the process is accessible via smartphone, tablet, or desktop, ensuring flexibility for participants and transparency for study teams. With TrialKit, eConsent is not just a feature. It’s a fully integrated part of a modern, participant-centric clinical trial ecosystem.
How eConsent Benefits Clinical Trials
For Participants
- Easier access and comprehension of consent forms
- Multilingual options and multimedia aids
- Remote access and increased flexibility
- Improved trust and transparency
For The Study Team
- Faster enrollment and reduced drop-offs
- Audit-ready digital trails
- Reduced errors and improved compliance
- Time and cost savings
Compliance and Security Standards
- HIPAA and GDPR alignment
- Regulatory compliance for global studies
- Data encryption and access controls



